Emerging cancer drugs may drive bone tumors
February 12, 2013
Emerging cancer drugs may drive bone tumors
By Julia Evangelou Strait
CHANG YANG, MD, PHD
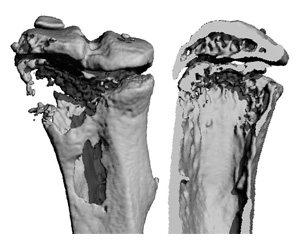
Investigational cancer drugs, IAP antagonists, may increase the risk of tumors spreading to bone. Tumors often cause bone loss, but IAP antagonist treatment accelerates the problem. The images show a bone with a tumor from a mouse treated with IAP antagonist BV6. The bone destruction is substantial, with gaping holes (left) and near total loss of the interior spongy bone (right).
Cancer drugs should kill tumors, not encourage their spread. But new evidence suggests that an otherwise promising class of drugs may actually increase the risk of tumors spreading to bone, according to researchers atWashington University School of Medicine in St. Louis.
The drugs, IAP antagonists, block survival signals that many cancer cells rely on to stay alive. Working in mice, the investigators found that targeting the same protein that makes tumors vulnerable to death also overactivates cells called osteoclasts, which are responsible for tearing down bone.
“These investigational drugs are getting broad attention right now because they seem to be very effective against primary tumors,” says senior author Deborah V. Novack, MD, PhD, associate professor of medicine. “There is also excitement because until now, these drugs have not appeared to have major side effects.”
The research appears in the February issue of Cancer Discovery.
In light of the study, Novack urges oncologists to think about protecting bone in patients taking IAP antagonists, including patients with cancers that don’t typically spread to bone. Numerous IAP antagonists are in early clinical trials against breast, lung, pancreatic, ovarian, prostate, liver, skin and blood cancers.
“For many of these cancers, doctors are not watching bone,” Novack says. “Osteoporosis is not the biggest concern when treating cancer, but if they’re not doing bone scans, they may miss a cancer spreading to bone.”
To maintain healthy bone, osteoclasts work in tandem with cells that build new bone. But IAP antagonists overactivate osteoclasts, destroying bone that is not replaced. In mice, the researchers showed that the drug led to osteoporosis, creating an environment that encouraged tumor growth in degrading bone, even while simultaneously killing breast cancer cells elsewhere.
After showing that the problem with IAP antagonists is specific to bone, Novack and her colleagues tested long-established drugs called bisphosphonates that inhibit osteoclasts and are used to treat osteoporosis.
“We found that bisphosphonate treatment protected bone from the negative effects of these drugs,” Novack says. “While bisphosphonates are common for breast cancer patients, they’re not, for example, commonly given to lung cancer patients. But since IAP antagonists are now in lung cancer trials, we’re saying doctors may want to consider bisphosphonate treatment for lung cancer or other cancer patients receiving these drugs. Or at least closely monitor the bone status.”
IAP antagonists are now only available to patients enrolled in phase 1 or 2 clinical trials. While these kinds of trials examine the short-term safety and effectiveness of new drugs, the researchers say they may not catch bone metastasis.
“These trials do not necessarily look for long-term effects of the drugs,” says Chang Yang, MD, PhD, staff scientist and the paper’s first author. “If the cancer is going to metastasize to bone, it may take six months to two years to see that outcome. This may not be seen during the clinical trial.”
Numerous drug companies are developing IAP antagonists intended for many kinds of cancer, but only Genentech agreed to provide Novack and her colleagues with its drug, called BV6, to evaluate in the study. Because the investigators could not obtain other proprietary IAP antagonists, they also made two other similar drug compounds and found them to have the same detrimental effects on the bone.
And to further ensure that over-stimulated osteoclasts are the only culprit in the bone metastasis associated with these new drugs, they performed studies in mice that lack the ability to dial up the production of osteoclasts. Even when given IAP antagonists, these mice were protected from osteoporosis and osteoclast activation.
Together, Novack says the studies have demonstrated that these results are unlikely to be a quirk of a particular compound.
“The osteoporosis and spread of tumors we see in bone are unintended side effects of IAP antagonists, but they’re not off-target effects,” she says. “They’re based on the mechanism of action for the entire class of drugs.”
Yang C, Davis JL, Zeng R, Vora P, Su X, Collins LI, Vangveravong S, Mach RH, Piwnica-Worms D, Weilbaecher KN, Faccio R, Novack DV. Anticancer IAP inhibition increases bone metastasis via unexpected osteoclast activation. Cancer Discovery. February 2013.
This study was supported by the National Institutes of Health (NIH), grant number AR052705, with additional support from AR52921 and AR53628, CA100730, and the Barnes-Jewish Foundation. Histological and microCT analysis was supported in part by the Washington University Center for Musculoskeletal Research NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), grant number AR057235. The Molecular Imaging Center was supported by NIH grant P50 CA94056. Genentech, Inc. provided BV6.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish andSt. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.

No hay comentarios:
Publicar un comentario